Fat Metabolism: Degradation
Physiological Pathways of Lipid Oxidation:

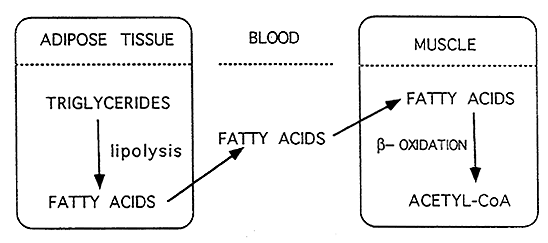
Lipolysis:
Hydrolysis of triacyglycerols to fatty acids and glycerol in the cytoplasm. Occurs primarily in adipose tissue but also in liver and muscle.
The enzymes of Lipolysis:
Hormone-Sensitive Lipase: catalyzes intracellular lipolysis
Lipoprotein Lipase: catalyzes hydrolysis of circulating triacylglycerols
Overview of Hepatic Fatty Acid Degradation:
FA = fatty acid
LPL = lipoprotein lipase
FABP = fatty acid binding protein
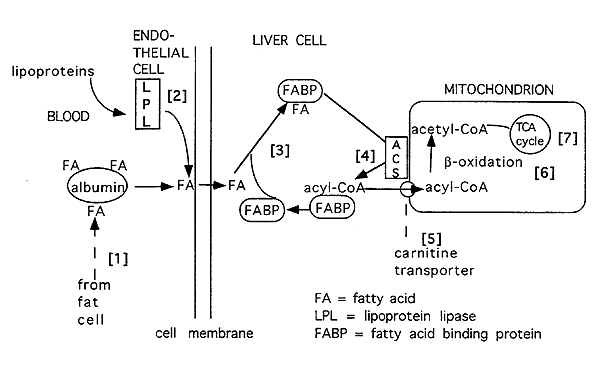
[1] Free fatty acids bound to albumin are released and delivered via the blood to tissues (i.e. liver).
[2] The lipoprotein system also delivers fatty acids to the liver and other tissues.
[3] Fatty acids w/in cell bound to fatty acid binding protein.
[4] Fats may also be derived by synthesis (lipogenesis) or breakdown of triacylglycerols or phospholipids. These fats are then activated to their acyl CoA form by acyl CoA synthetase (ACS).
[5] Once in an "acyl CoA" form it is converted into a carnitine derivative for transport into the mitochondria.
[6] It is then subjected to b-oxidation.
[7] Acetyl CoA produced by b-oxidation feeds into the citric acid cycle for energy production.
Mitochondrial Uptake and b-Oxidation of Fatty Acids
Carnitines Role in Mitochondrial Fatty Acid Transport:
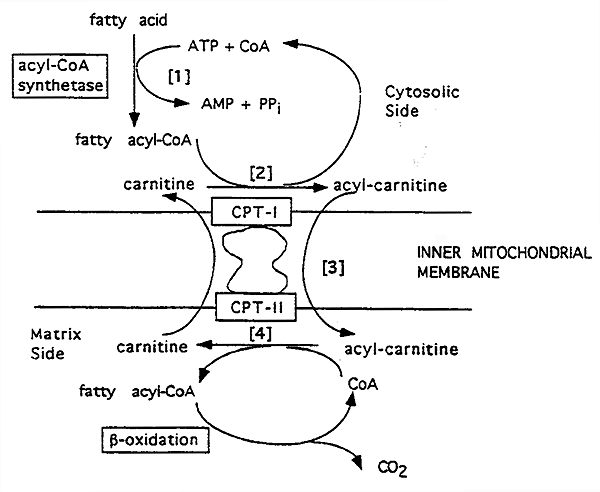
[1] The product of acyl CoA synthetase, (ACS) long-chain acyl CoA, cannot pass through the inner mitochondrial membrane.
[2] So, it is transformed by carnitine palmitoyl transferase I (CPT-I) to acylcarnitine. (-) Malonyl CoA (lipogenesis)
[3] Carnitine-acylcarnitine translocase acts as a membrane carnitine "exchange transporter".
[4] Acylcarnitine goes in and a carnitine comes out.
The acylcarnitine reacts with CoA via carnitine palmitoyl transferase II (CPT-II), attached to the inner membrane.
Acyl CoA is reformed in the mitochondrial matrix and carnitine is liberated.
Deficiencies in CPT's lead to considerable muscle weakness, as fatty acids are a major fuel for muscles.
Fatty Acid Activation and Transport
b-Oxidation of Free Fatty Acids With an Even Number of Carbons:
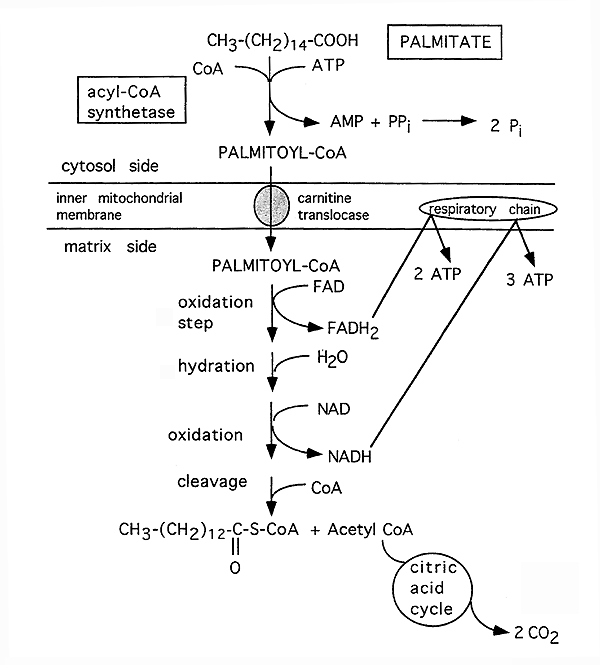
Reactions 2 through 5 recycle to remove consecutive 2-carbon units. On the final cycle, 2 acetyl CoA molecules are formed. Thus, a 16 carbon fatty acid needs to cycle only 7 times. The first 12 carbons are released during cycles 1 through six and the seventh and final cycle produces 2 Acetyl CoA, i.e. the final 4 carbons.
Energy Production From One Molecule of Palmitic Acid (16 carbons) :
1 NADH = 3 ATP
1 FADH2 = 2 ATP
1 Acetyl CoA = 12 ATP
35 ATP (7 cycles b-oxidation, 7 NADH and 7 FADH2 produced)
72 ATP (6 cycles b-oxidation, 6 Acetyl CoA entering the TCA cycle)
2 Acetyl CoA: 24 ATP (produced in the last step from the remaining 4 carbons, enters TCA cycle)
Total: 131 ATP
Odd Chain Fatty Acids:
Endproducts are Propionyl CoA and Acetyl CoA caused by cleavage of a 5-carbon fatty acid during the final cycle. Acetyl CoA goes into the TCA cycle while the Propionyl CoA must be converted into Succinyl CoA.
Conversion of Propionyl CoA to Succinyl CoA:
Propionyl CoA Carboxylase:
Propionyl CoA + ATP + CO2 -----> Methylmalonyl CoA + AMP + PPi
Methylmalonyl CoA Mutase:
Methylmalonyl CoA -----> Succinyl CoA (enters Citric Acid Cycle)
Energy Production:
1 Sucinyl CoA = 6 ATP
35 ATP (7 cycles b-oxidation, 7 NADH and 7 FADH2 produced)
72 ATP (6 cycles b-oxidation, 6 Acetyl CoA entering the TCA cycle)
Acetyl CoA + 1 Succinyl CoA 18 ATP (produced in the last step from the remaining 5 carbons, enters TCA cycle)
Total: 125 ATP
-1 ATP (used in the Propionyl CoA Carboxylase reaction) = 124 ATP
Comparison of Fatty Acid b-Oxidation and Synthesis

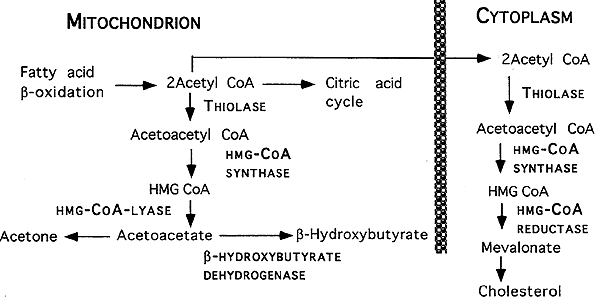
Ketone Metabolism (Ketogenesis):
Occurs in the liver when Acetyl CoA production exceeds the limits of its oxidation in the citric acid cycle. This might occur in starvation or uncontrolled diabetes.
Conditions Favoring Ketoacidosis (ketogenesis):
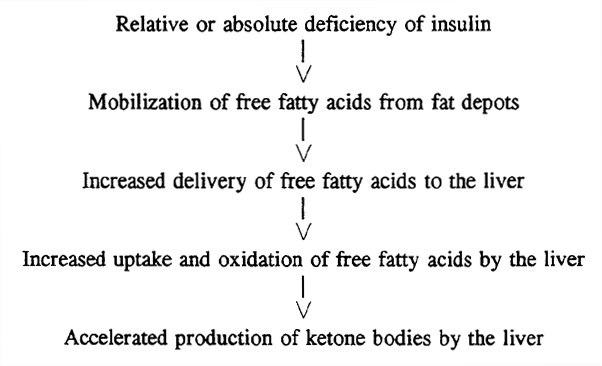
Ketone Body Formation in Liver:
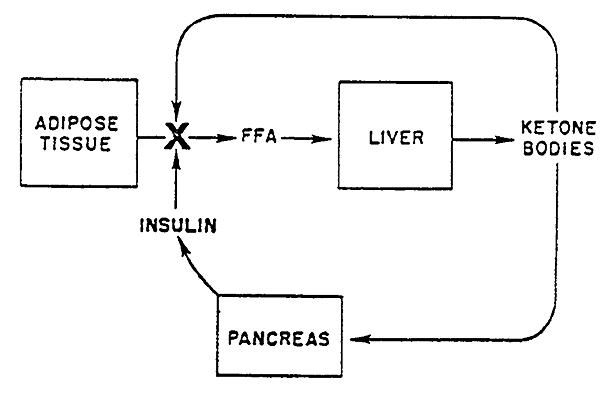
Normal Prevention of Ketoacidosis:
Insulin whose release is promoted by ketone bodies, inhibits lipolysis to decrease the supply of fatty acids (Acetyl CoA) and thus curtail ketogenesis preventing ketoacidosis
Ketone Body Oxidation: long-term starvation or ketoacidosis
Tissues that can use ketones as "fuel": brain, muscle, kidney, intestine
b-hydroxybutyrate + NAD+ ---> NADH + acetoacetate
acetoacetate + succinyl CoA ---> acetoacetyl CoA + succinate
acetoacetyl CoA + CoA ---> 2 acetyl CoA
© Dr. Noel Sturm 2019